A Lifesaving Implant for Thoracic Insufficiency
 Robert Campbell
|
Harland-Smith lecturer Robert Campbell,
Director of the Center for Thoracic
Insufficiency Syndrome, Children's
Hospital of Philadelphia, began his talk
on surgical anatomy by telling the orthopaedic
residents - "forget everything you know about scoliosis". He then
discussed the anatomical basis of disease, an innovative device based on a
fresh anatomical perspective, and the tribulations of surgical innovation at the
patient and the FDA level. He ended by quoting the pioneer surgeon John
Cobb who said in 1958: "In the future study of scoliosis, it will be necessary
to keep our eye on the patient and not on the curve".
Bob is globally recognized for developing the VEPTR, the vertical expandable
prosthetic titanium rib, which is a lifesaving implant for young children with severe
deformities of the spine and chest wall. Andrew Howard had the opportunity to
discuss surgical innovation with Bob and hear the advice of a master innovator.
Q - Dr. Campbell, where did the idea for this device come from?
A - It starts with a patient. He was six months old and dying on a ventilator in the
ICU from thoracic insufficiency. Had been to Austin, to Houston, had a tracheotomy,
frequently arrested. Interestingly the standard approach then was something
called a Toronto chest wall splint. That didn't work. He needed more chest volume
so he could aerate his lungs. We planned an opening wedge thoracotomy but I
needed a device to expand the chest wall - to hold it open. I sat down the night
before and planned an improvised solution using Steinmann pins bent around the
ribs. Physiologically, it worked. Technically, it was very difficult. I knew we needed
to revise our implant as the baby grew - so the device was
invented just for this youngster. I was motivated because I
knew I was doing the revision myself. I found out later how
many orthopaedic surgeons had turned this patient down!
Q - So you designed a device?
A - There are three crucial points to innovation. The
device comes last. The critical first point is to reduce the
problem to an anatomic and mechanistic understanding.
The second is to come up with a surgical approach
to that anatomical problem. It is only at the final stage
that we design the device. In this child's case we need a 3
dimensional functional appreciation of the problem. It's
not a bent spine that needs straightening. The problem
is low chest volume and an absent chest well - which
makes the pump mechanism of the diaphragm ineffective.
The surgical solution is an expansion thoracoplasty
- like an opening wedge between the ribs. And the device
has to be a better, safer means of creating and maintaining
that volume. So I designed the VEPTR to do that.
Q - How long did it take to design the device?
A - The first VEPTR took me 18 months of concentrated
work. Two or three hours a day, plus travel, probably
2000 hours of my time in total. I considered it a
Manhattan project - we had this revision surgery coming
up. I had the advantage of two years of engineering
school - so I had some basics. I could design the joints
and do all my own drawings - to the point that they
are ready for manufacture. Make sure these are signed,
notarized, dated, detailed. These days you can send them
to a technician and create computerized models and templates
very quickly.
Q - And then you had it manufactured?
A - It's never easy. I remember talking to Zimmer, an
orthopaedic giant, early on. When they asked me to estimate
the market size, I said 'one kid'. They lost interest
pretty quickly. I found a company called Techmedica,
whose main business was custom hip and knee implants.
When they heard the patient story they agreed to manufacture
the device as a one off in return for publicity. I
insisted that the publicity had to be tasteful - only at
six weeks postop - only with the patient well - so we
scheduled the surgery.
Q - Any other hurdles to deal with?
A - Oh yes. Just before surgery - the device was not ready
- the fit between expanding components was not precise,
and the engineers wanted to add a plastic bushing. I didn't
like that idea, because of wear debris. I wanted to convert a
rectangular cross section to a t-shape. I cancelled the clinic
and flew out to Techmedica to meet three engineers and
the CEO in the boardroom. The engineers all told me a
t-shaped slide was too complex to machine. By then I knew
the company a bit and I asked them to call in Malcolm,
a lathe worker, from the shop floor. He came into the
boardroom in his bib overalls and I showed him my revised
drawings and asked if he could machine it. He said yes, and
the CEO backed the lathe worker. They worked four weeks
day and night to have it ready - it worked for that patient
and has been the basis of the design ever since.
Q - How many patients now?
A - About 500 patients of my own, and growing by 5%
to 10% every year. The VEPTR is used all around the
world - over 4000 have been implanted.
Q - What about regulatory approval?
A - Before we did the first one, we went to the local institutional
review board. They approved a custom device for
a lifesaving indication. The case went well, the company's
publicist did a good job - 125 newspapers and national TV
coverage - this was in 1989 before the internet - I was even
reading about it in supermarket tabloids. So the floodgates
opened then and patients from everywhere showed up. The
second patient was approved by the IRB. The third patient
- I was at the review board for this one, the sister, the
nun, on our hospital's IRB looked me in the eye and said
"Dr. Campbell, the jig is up." She couldn't approve a third
similar device as unique, as a one off - and she was right. I
called the company and said we need to talk to the FDA.
I called the FDA and left a message. I had just lit a fuse.
|
Q - What is the FDA story?
A - A nice guy from the FDA called me back in the
middle of a busy clinic. Turns out it was Dr. Thomas
Callaghan - the chief of medical devices. He was a biologist
from Yale and he really liked kids. Pretty soon I was
meeting him - holding the Xray up to the light - showing
him the VEPTR - he asked "Do these kids die?" and
I said yes, so he said "then let's go ahead with that".
|
"Anyone who wants help navigating the FDA for a
surgical device, contact me."
|
Q - And how many years to full approval with that
encouraging start?
A - Fourteen years. About 10,000 Bob Campell hours.
Q - Worth it?
A - Absolutely.
Q - Other twists and turns?
A - Of course. Techmedica closed in 1994 and I ended
up with 800 pounds of manufacturing dies in my office
and 30 kids with devices in. That is where Synthes picked
up the product and they continue with it. Synthes helped
us with the multicentre premarket trial from the 1990s on.
In 2002 the FDA asked for control patients, for the first
time. We had to challenge this as unethical - this is a fatal
condition for some of these kids. We submitted historical
controls gathered through the scoliosis research society. This
was rejected by the FDA. The premarket approval application
was withdrawn, and the application resubmitted as a
humanitarian device exemption. That means establishing
proven safety and probable benefit, when treating a potentially
fatal condition. That is how VEPTR is approved in
the US. In other countries the indications are not so tight.
Q - Where are things today?
A - A very exciting stage. I moved from San Antonio to
CHOP (Children's Hospital of Philadelphia) two years
ago. Our new centre of excellence at CHOP has 16
physicians including cardiovascular surgeons, geneticists,
pulmonologists, general surgeons, otolaryngologists, and
more. I am the referee and all ideas are welcome. We
are using 3D MRI and other technologies to refine our
understanding of thoracic function; and working on new
operations and new devices.
Q - Advice?
A - Perseverance. I always had a very powerful guardian
angel in the background. I always looked for internal
champions - in my university, at the company, at the FDA.
Q - Final Words?
A - I mean this. Anyone who wants help navigating the
FDA for a surgical device, contact me. I am ready to help
any surgeon with that. I have learned a lot.
Andrew Howard
Figure 1
The Vertical Expandable Prosthetic Titanium Rib (VEPTR) device. It can be
configured to span from rib to rib (1a), from rib to spine (1b.) or from rib
to pelvis (1c.)
 Figure 1a
|
 Figure 1b
|
 Figure 1c
|
Figure 2
A radiograph showing the device in a patient. It expands the chest wall,
and can be extended multiple times as the child grows, with an outpatient
surgical procedure. 2a - preoperative. 2b - postoperative. Note the
opening wedge thoracotomy creating a much higher thoracic volume on
the left, concave, side.
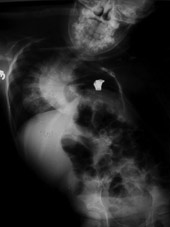 Figure 2a
|
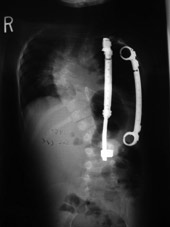 Figure 2b
|
|